Nomenclature
The large and heterogeneous mixture of proteins forming the extended BCL-2 family poses major challenges to researchers trying to elucidate structure-function relationships and gain mechanistic insights into their biological roles. As part of the BCL2DB initiative, we propose a re-classification of the proteins related or connected to BCL-2 into a large protein group divided into three subgroups: BCL-2 homologs, viral BCL-2-related proteins and BH3-containing proteins with no homology to BCL-2.
BCL-2 homologs
| BCL-2 homologous proteins share a similar α-helical bundle fold and can possess up to four different regions of sequence similarity termed BCL-2 Homology (BH) motifs (BH1-BH4). The cellular BCL-2 homologs has a typical globular structure with 5-7 amphipathic α-helices forming an envelope around a core helical hairpin (α5-α6 in BAX and BCL-2). Sequence composition in BH motifs is variable among the different BCL-2 homologous proteins, ranging from one (e.g. BID) to four (in proteins such as BCL-2 and BCL-xL). BCL2DB therefore favors a strategy based on profile-HMMs to detect BCL-2 homologous sequences. Phylogenetic studies reveal that the subclass of cellular BCL-2 homologs originated very early in metazoan history and diversified through waves of gene duplications. In vertebrates, BCL-2 homologous proteins usually fall into one of three clades called the BCL-2-like, BAX-like and BID-like subfamilies. Duplications in early branching metazoan species and other invertebrates gave rise to a rich repertoire of paralogs, some of them being lineage-specific (e.g. Drosophila BUFFY and DEBCL). Strikingly, a number of viral genomes appear to encode proteins with clear sequence similarity to BCL-2. Expected structural similarity to BCL-2 was formally documented for a handful of these viral BCL-2 homologs (e.g. the BHRF1 EBV protein). The viral genes of the BCL-2 family do not form a clade, i.e., most of them were acquired independently by different virus species from their individual hosts. Cellular and viral BCL-2 homologs form the core BCL-2 family. | 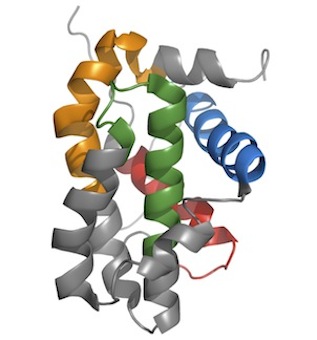 Human Bcl-2 protein structure. The structure has been determined by NMR spectroscopy (PDB:1g5m; Petros et al., 2001). This globular protein consists of a bundle of 6 α-helices with 2 central hydrophobic α-helices surrounded by 4 amphipathic α-helices. The BH4, BH3, BH1 and BH2 motifs are in red, blue, green and orange, respectively. Human Bcl-2 protein structure. The structure has been determined by NMR spectroscopy (PDB:1g5m; Petros et al., 2001). This globular protein consists of a bundle of 6 α-helices with 2 central hydrophobic α-helices surrounded by 4 amphipathic α-helices. The BH4, BH3, BH1 and BH2 motifs are in red, blue, green and orange, respectively. |
Viral BCL-2-related proteins
| In addition to viral BCL-2 homologs, a number of other viral proteins with reported or expected structural resemblance to BCL-2 in the absence of detectable sequence similarity have been catalogued (e.g. the vaccinia virus F1 protein); however, phylogenetic homology to BCL-2 (common ancestry) cannot be stated with confidence for these members, prompting their classification as a separate group (structurally related BCL-2-like fold proteins). |
BH3-containing proteins with no homology to BCL-2
| Within this class, the BH3-only subclass include eight members that have well-studied roles in the apoptotic network of protein-protein associations. Depending on their action mode, these classical BH3-only members were subdivided into activators and sensitizers. Moreover, numerous other BH3-containing proteins have been reported as having a functional BH3 motif in their sequence. The BH3 peptide allows interactions between BCL-2 homologs or between BCL-2 homologs and the BH3-only proteins. |
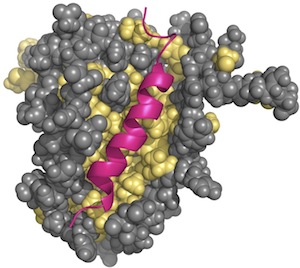 | Interaction between a BCL-2 homolog and a BH3 peptide. The structure has been determined by NMR spectroscopy (PDB:1g5j; Petros et al., 2000). This Bcl-xL protein shows an elongated hydrophobic cleft presents at the protein surface formed by hyprophobic residues (in yellow). This cleft serves as the binding site for the BH3 peptides of interacting proteins. In this structure, the Bad BH3 peptide (in pink) binds in the cleft. |
References
Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators.
Aouacheria A., Brunet F., Gouy M.
Mol Biol Evol., 2005, 22(12):2395-2416.
Evolution of Bcl-2 homology motifs: homology versus homoplasy.
Aouacheria A., Rech de Laval V., Combet C., Hardwick JM.
Trends Cell Biol., 2013, 23(3):103-111.
A poxvirus Bcl-2-like gene family involved in regulation of host immune response: sequence similarity and evolutionary history.
González JM., Esteban M.
Virol J., 2010, 7:59.
Multiple functions of BCL-2 family proteins.
Hardwick JM., Soane L.
Cold Spring Harb Perspect Biol., 2013, 5(2).
SnapShot: BCL-2 proteins.
Hardwick JM., Youle RJ.
Cell., 2009, 138(2):404-404.e1.
Solution structure of the antiapoptotic protein bcl-2.
Petros AM, Medek A., Nettesheim DG., Kim DH., HS., K., Matayoshi ED., Oltersdorf T., Fesik SW.
Proc Natl Acad Sci U S A., 2001, 98:3012-3017.
Structural biology of the Bcl-2 family of proteins.
Petros AM, Olejniczak ET., Fesik SW.
Biochim Biophys Acta., 2004, 1644(2-3):83-94.
Intrinsically disordered proteins in bcl-2 regulated apoptosis.
Rautureau GJ., Day CL., Hinds MG.
Int J Mol Sci., 2010, 11(4):1808-1824.
